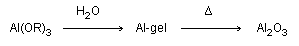
- Research
in the Barron Group
-
-
- Alumina Nanoparticles:
from Sol-Gel to Composites to Hybrid
Materials
- Alumina
sol-gels
- What is an alumina
sol-gel or alumoxane?
- Understanding
Structure
- Developing a Rational
Synthesis
- Alumoxane
Nanoparticles
- Why are carboxylates
ideal ligands?
- Applications of
Carboxylate-Alumoxanes as Ceramic Precursors
- Ceramic Processing
and Composites
- Rational Control
over Ceramic Pore Size
- Chemically Functionalized
Nanoparticles
- Inorganic Organic
Hybrid Materials
- Catalyst
Components
Top of
Page
- Alumina
Sol-Gels
-
- What is an Alumina Sol-Gel or
Alumoxane?
-
- The common solution-gelation route to
aluminum oxides employs aluminum hydroxide or hydroxide-based
material as the solid colloid, the second phase being water and/or
an organic solvent. Aluminum hydroxide gels have traditionally
been prepared by the neutralization of a concentrated aluminum
salt solution; however, the strong interactions of the freshly
precipitated alumina gels with ions from the precursors solutions
makes it difficult to prepare these gels in pure form. To avoid
this complication alumina gels may be prepared from the hydrolysis
of aluminum alkoxides, Al(OR)3.
-
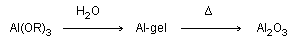
-
- Although this method was originally
reported in 1922, it was not until the 1970's that alumina
aerogels were prepared, and transparent ceramic bodies were
obtained by the pyrolysis of suitable alumina gels, that interest
increased significantly. There have been several efforts to
improve the processing control of sol-gels (including development
of environmentally benign routes), however, we proposed that
without an understanding of the structure of these materials any
further development was limited.
-
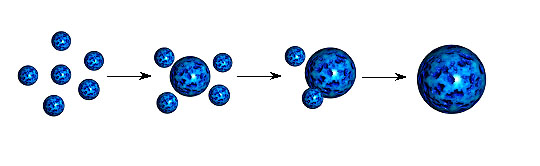
- The aluminum based sol-gels formed during
the hydrolysis of aluminum compounds belong to a general class of
compounds: alumoxanes. Alumoxanes were first reported in 1958,
however, have since been prepared with a wide variety of
substituents on aluminum. The structure of alumoxanes
was proposed to consist
of linear or cyclic chains (i.e., analogous to that of
poly-siloxanes).
- Understanding Structure
-
- In order to determine the structure of an
alumina sol-gel we re-investigated the first sol-gel reaction; the
hydrolysis of Al(OSiEt3)3. Using a
combination of 1H, 2H, 13C,
17O, 27Al, and 29Si NMR
spectroscopy and XPS we proposed the structure of the
siloxy-alumoxanes to consist of a Al-O core
whose structure was that of the mineral
boehmite, [Al(O)(OH)] (Chem. Mater., 1992,
4, 167). This was confirmed by the X-ray structural
characterization of
Al10(OH)16(OSiEt3)14.
-
- Top of Page
- Developing a Rational
Synthesis
-
- Precursor sol-gels are traditionally
prepared via the hydrolysis of aluminum compounds. This
"bottom-up" approach of reacting small inorganic molecules to form
oligomeric and polymeric materials has met with varied success,
due to the difficulties in controlling the reaction conditions,
and therefore the stoichiometries, solubility, and processability,
of the resulting gel. It would thus be desirable to prepare
alumoxanes in a one-pot bench-top synthesis from readily
available, and commercially viable, starting materials, which
would provide control over the products. Based on our knowledge of
the boehmite-like core structure of hydrollytically stable
alumoxanes, we posed the following question: Can alumoxanes be
prepared directly from the mineral boehmite? At that time, a
"top-down" approach represented a departure from the traditional
synthetic methodologies.
-
- In the siloxy-alumoxanes we had shown the
"organic" unit itself contains aluminum. Thus, in order to prepare
the siloxy-alumoxane similar to those we have previously reported,
the anionic moiety, the "ligand"
[Al(OH)2(OSiR3)2]-,
would be required as a bridging group; adding this unit would
clearly present a significant synthetic challenge. However, the
carboxylate-alumoxanes represent a more realistic synthetic target
since the carboxylate anion,
[RCO2]-, is an isoelectronic and
structural analog of the organic periphery found in our
siloxy-alumoxanes. Based upon this rational we have developed a
"top-down" approach based upon the reaction of boehmite,
[Al(O)(OH)]n, with carboxylic acids (J.
Mater. Chem., 1995, 5, 331). This synthesis has been
extended to allow for aqueous processing (Chem. Mater.,
1997, 9, 2418).
-
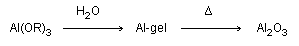
- The carboxylate-alumoxane materials
prepared from the reaction of boehmite and carboxylic acids are
air and water stable materials and are very processable. The
soluble carboxylate-alumoxanes can be dip-coated, spin coated, and
spray-coated onto various substrates. The physical properties of
the alumoxanes are highly dependent on the identity of the alkyl
substituents. The alumoxanes are
indefinitely stable under ambient conditions, and are adaptable to
a wide range of processing techniques. Additional advantages
include: the low price of boehmite and the availability of an
almost infinite range of carboxylic acids.
Top of Page
- Alumoxane Nanoparticles
-
- A detailed study of the
carboxylate-alumoxanes shows them to be aluminum-oxide
nanoparticles whose surface is stabilized by the carboxylate
group. The size of the nanoparticle is dependent on the identity
of the carboxylate and the solution pH (J. Non-Cryst. Solids,
2001, in press).
-
- The majority of our studies use the
following carboxylic acids: acetic (A-H), methoxyacetic (MA-H),
methoxyethoxyacetic (MEA-H), methoxyethoxyethoxyacetic (MEEA-H),
para-hydroxybenzoic (p-HB-H) and lysine (L-H).
-
- We have been able to model the
surface of the alumoxane nanoparticles to confirm the mode of
binding of the carboxylate groups
(Organometallics, 1995, 14, 4026).
- Why are carboxylates ideal
ligands?
-
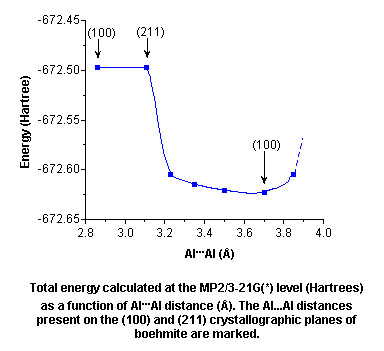
- Using a combination of X-ray
crystallography and ab initio calculations (Organometallics,
1997, 16, 329) we have shown that the carboxylate
ligand is therefore near perfectly suited to bind to the (100)
surface of boehmite (Al...Al = 3.70 Å), and hence
stabilize the boehmite-like core in carboxylate
alumoxanes.
-
- We have extended the principle of
using model compounds to other inorganic surfaces (Polyhedron,
1998, 17, 3121).
-
-
Top
of Page
- Applications of Carboxylate-Alumoxanes
as Ceramic Precursors
-
- Ceramic Processing and
Composites
-
- The carboxylate-alumoxane nanoparticles are
ideally suited to a wide range of ceramic processes. We have
demonstrated examples of the following: use of the alumoxanes as
pre-ceramic binders for low shrinkage green bodies (Chem.
Mater., 1997, 9, 2418); the infiltration and surface
repair of ceramic surfaces; the dip coating of SiC, sapphire,
graphite and Kevlar fibers (J. Mater. Res., 2000,
15, 2228) to be used to simplify the fabrication of
FRCMCs.
-

- Possible applications
of these process improvements are in the automotive and aerospace
industries (World Car Conference '96, University of
California, Riverside, p. 151).
-
- Top of Page
-
- Rational Control over Ceramic Pore
Size
-
- Carboxylate-alumoxanes are converted to
alumina upon thermolysis. The pore size and pore size distribution
is influenced by the selection of the organic substituent on the
nanoparticle surface, while the average pore sizes may be altered
through either physical or chemical mixtures of two (or more)
carboxylate-alumoxanes. Most important is our ability to create
intra-granular porosity of a controlled size (Adv. Mater.,
2000, 12, 734).
-

Top of Page
- Our ability to produce alumina with small
pore size and very narrow pore size distribution has allowed us to
fabricate alumina ultrafiltration membranes derived from
carboxylate&endash;alumoxane nanoparticles (J. Membrane Sci.,
2001, in press). These membranes have a molecular weight
cut-off in the range of 30,000 g.mol-1 and permeability compare
favorably (or are superior to) commercially
available ultra filtration membranes.
-
-

Top of Page
Chemically Functionalized
Nanoparticles
- Given the wide range of carboxylic acids
available with secondary functionalization it is possible to
simply prepare alumina nanaoparticles with
well designed chemical functionalization on the
exterior.
- Inorganic-Organic Hybrid
Materials
-
- Chemically functionalized alumina
nanoparticles (carboxylate-alumoxanes) are used as the inorganic
component of a new class of inorganic-organic hybrid materials.
Lysine- or para-hydroxybenzoic acid-derivatized alumoxanes.
The peripheral organic hydroxides and amines of these
carboxylate-alumoxanes either react directly with epoxide resins,
such as the diglycidyl ether of bisphenol-A (DER 332), to form a
hybrid material, or in the presence of an organic resin and
hardener system to form a composite material (Chem. Mater.,
2000, 12, 795). The properties and cure times of the
alumoxane hybrid and composite materials are distinct from both
the pure resins and from a physical blend of the resins with
traditional ceramic fillers. A significant increase in thermal
stability and tensile strength is observed for both the hybrid and
composite resin systems.
-
- The presence of the
alumoxane nanoparticles chemically bound to the resin results in a
drastic improvement in the torsional strength of composites as
well as smaller increases in tensile strength and thermal
stability.
-
- Top of Page
- Catalyst Components
-
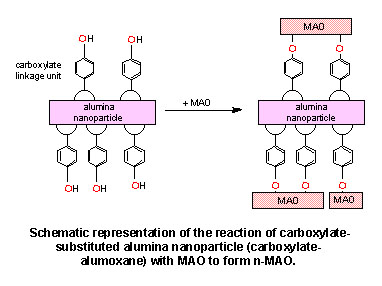
- A new class of metallocene/MAO-based solid
olefin polymerization catalyst has been developed using chemically
functionalized nanoparticles (carboxylate-alumoxanes) as a well
defined substrate. Reaction of para-hydroxybenzoate-alumoxane
(p-HB-A) nanoparticles, formed from the reaction of the
acid with boehmite, with methylalumoxane (MAO) results in a solid
nanoparticle-based MAO (n-MAO) which reacts readily with
zirconocenes, including: Cp2ZrCl2,
Cp2ZrMe2 and
(nBuCp)2ZrCl2, to make an active
solid catalyst for olefin polymerization. The catalytic activity
of the n-MAO based catalyst is greater than the homogeneous analog
under similar Al(MAO):Zr ratio and is comparable to
that of a traditional silica supported catalyst but offers the
potential of being easily chemically modified.
Top
of Page
Return
to Research in the Barron Group
Return
to Barron Group Home Page