-
Research
in the Barron Group
- Group 13
Organometallic and Coordination Compounds
-
-
-
- Group 13 Alkyls:
Structure, Properties and Reactivity
-
- Structure and
Bonding
- Control over
Volatility
-
- Reactivity
- Oxidation
- Hydrolysis
- Ligand
Exchange
- Siloxane
Cleavage
-
- Lewis Acid Properties of
Group 13 Metal Compounds
- Activation of Small
Molecules
- Activation of
Metals
-
- Alumoxanes
- Redefining their
Structure
- Redefining their Mode of
Activation: Latent Lewis Acidity
- New
catalysts
-
- Group 13 Compounds as
Ligands
Group 13 Alkyls: Structure, Properties and
Reactivity
- The Group 13 alkyl compounds, in particular
those of aluminum, are widely used in industrial catalysis,
organic synthesis and the electronics industry. As would be
expected from their position in the Periodic Table, they have the
general formula, MR3-nXn, where R is a
hydrocarbon unit and X can be a hydride, halide, alkoxide, or
related group.
-
- Aluminum and indium trialkyls are
ordinarily oligomeric involving alkyl bridges, except where
precluded by steric interactions. Trimethyl aluminum is the
archetypal electron deficient compound, while the presence of
halide, alkoxide, amide and similar groups results in oligomers
with octet configurations. In contrast to the aluminum and indium
compounds GaR3 are monomeric, although the compounds of
the formula, GaR3-nXn follow the same
patterns as their aluminum analogs.
-
- The most common reaction of Group 13 alkyls
involves the reaction between the M-R bonds and an acid protic
source, HX. However, M-R bonds also undergo insertion reactions
involving a variety of small molecules.
-
- Top of
Page
Structure and Bonding
- Our studies into the basic structure and
bonding in aluminum compounds involve a desire to understand the
relative magnitude of steric versus electronic effects in defining
the structures of simple organometallic compounds
(Organometallics, 1991, 10, 597) as well as
controlling the extent of oligomerization (J. Chem. Soc.,
Dalton Transs., 992, 3179). Using a simple series of phosphine
adducts of AlMe3 we were able to demonstrate that
steric factors predominated (J. Chem. Soc., Dalton Trans.,
1988, 3047), however, based upon structural
(Organometallics, 988, 7, 2543), spectroscopic
(J. Am. Chem. Soc., 1990, 112, 3369) and ab initio
(J. Am. Chem. Soc., 1991, 113, 39) studies we
proposed the presence of a weak p-type
interactions between aluminum and oxygen in monomeric aluminum
alkoxides and aryloxides involving the donation of electron
density of the oxygen lone pair to the Al-X anti bonding
orbital.
-

- Our evolving understanding of the structure
and bonding in Group 13 organometallic compounds has led to the
development of a quantitative measure of steric bulk
(Organometallics, 1999, 18, 4399), and the
development of a Lewis acidity scale.
-
- Other results include: the synthesis of the
first 6-coordinate aluminum alkyl (J. Am. Chem. Soc., 1989,
111, 398); the first observation of the trans-influence in
an aluminum compound. (Organometallics, 1989, 8,
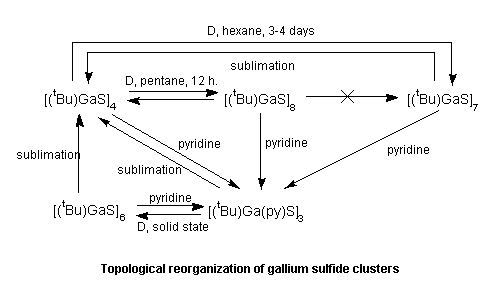
1828); Topological reorganization of gallium sulfide clusters
(Organometallics, 1992, 11, 2783).
-
- Top of
Page
Control over Volatility
- Industrial MOCVD processes rely almost
exclusively on gaseous and liquid precursors. Systems where solid
precursors are employed are generally undesirable due to the
difficulty of maintaining a constant flux of source vapors over a
non-equilibrium percolation (solid) process (Adv. Mater. Optic.
Electron., 1993, 2, 271). Since the majority of
metal-organic compounds reported in the literature are solids,
alternative approaches have been used to overcome these
difficulties including the synthesis of new more volatile
precursor compounds. Delay in developing a rational approach to
volatile compounds is, in part, due to a lack of a detailed
understanding in the factors that control the volatility of
metal-organic compounds.
-
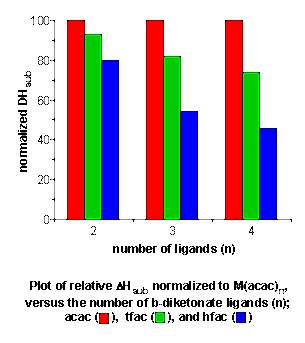
- Temperature of volatilization and
sublimation enthalpies (DHsub)
for cubane compounds [(R)Ga(E)]4 (where R =
tBu, EtMe2C, Et2MeC, or
Et3C and E = S, Se, or Te) have been determined
(Chem. Mater., 1997, 9, 796). The temperature of
volatilization was found to increase in a linear fashion with
respect to increasing molecular mass, perturbations were observed
that can be attributed to intermolecular ligand interactions.
Sublimation enthalpies (DHsub)
for each cubane are more dependent on the degree of branching of
the alkyl ligand than the molecular mass effects alone. Using the
TGA sublimation data vapor pressures may be calculated for each of
the cubane compounds over a wide range of
temperatures.
-
- Sublimation enthalpies (DHsub)
for M(b-diketonate)n
complexes were also shown to be dependent on the number and type
of intermolecular interactions, rather to be more substantial than
molecular mass effects. The relationship between the
DHsub
of the substituted b-diketonate
derivatives as compared to the values for parent
M(acac)n may be used to predict either quantity for a
range of M(b-diketonate)n
complexes where the values for M(acac)n are known
(Adv. Mater. Optics Electron., 2000, 10,
223).
-
Top of
Page
Reactivity
- The types of reactions that Group 13
compounds undergo (oxidation, hydrolysis/solvolysis, ligand
exchange, etc.) are well known, however, a detailed understanding
of the mechanisms, intermediates and product structures is often
missing. We have attempted to gain a fundamental understanding of
the reactivity of Group 13 organometallic compounds and apply our
understanding across a wide range of applications: from surface
stabilization of nanoparticles to the chemical control over the
structure of a solid material.
Oxidation
- The pyrophoric nature of Group 13 trialkyls
(MR3) means that oxidation reactions are often
uncontrolled if not catastrophic! However, if the steric bulk of
the alkyl group (R) is sufficient it is possible to isolate the
intermediates. For example, reaction of In(tBu)3
with dioxygen results in the isolation of
[(tBu)2In(OOtBu)]2
(J. Am. Chem. Soc., 1989, 111, 8966). The gallium
analog is made in a similar manner and their use as mild oxidation
agents has been explored (Organometallics, 1993, 12,
4908).
-
- In a related series of reactions, the
interaction of Ga(tBu)3 with elemental
sulfur, selenium, and tellurium was explored (Organometallics,
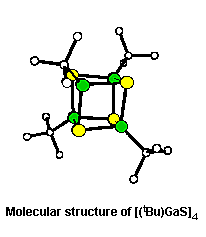
1992, 11, 1055). In the case of sulfur the
alkyldisulfide derivative,
[(tBu)2Ga(SStBu)]2,
is formed as an intermediate to the
[(tBu)Ga(S)]4 cubane. For selenium
and tellurides the cubanes are formed via
[(tBu)2Ga(EtBu)]2
(E = Se, Te).
Top of
Page
Hydrolysis
- The hydrolysis of aluminum alkyls is
ubiquitous in the handling of these compounds. Partial hydrolysis
is the most common cause of impurities, however, the purposeful
hydrolysis of AlR3, to alkylalumoxanes is a vital part
of the new generation of highly active polymerization
catalysts.
-
- The hydrolysis of
Al(tBu)3 yields the trimeric hydroxide
compound, which may be converted to a mixture of alumoxanes,
[(tBu)AlO]n (n = 6, 7, 8, 9, 12)
upon mild thermolysis. Based on spectroscopic evidence, and
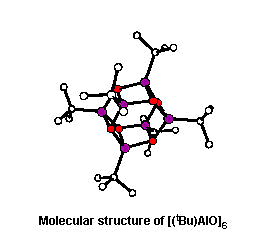
confirmed by the X-ray crystallographic structural determinations
of [(tBu)AlO]6,
[(tBu)AlO]8 and
[(tBu)AlO]9, we have shown that
these compounds have three-dimensional cage structures (J. Am.
Chem. Soc., 1993, 115, 4971 and Organometallics,
1994, 13, 2957). In addition, we have demonstrated that
partial hydrolysis of Al(tBu)3 allows for
the isolation of the tetra-alumoxane,
[(tBu)2Al{OAl(tBu)2}]2,
whose structure contains the three-coordinate aluminum center that
has been proposed to be active in olefin
polymerization.
-
- The oxidation and hydrolysis of
(Me2InPPh2)2 to yield the first
13/16 cubane compound (Polyhedron, 1988, 7, 2091).
Related to hydrolysis is the reaction of
Ga(tBu)3 with H2S which yields
the first gallium hydrosulphido complex
[(tBu)2Ga(SH)]2,
thermolysis of which results in the formation of the first Ga-S
analog of an alumoxane, [(tBu)GaS]4
(J. Chem. Soc., Chem. Commun., 1991,
1315).
Ligand Exchange
- While ligand exchange of alkyls, alkoxides,
and halides is common for Group 13 compounds, we have discovered a
chalcogenide exchange unique to gallium-telluride
cubanes.
-
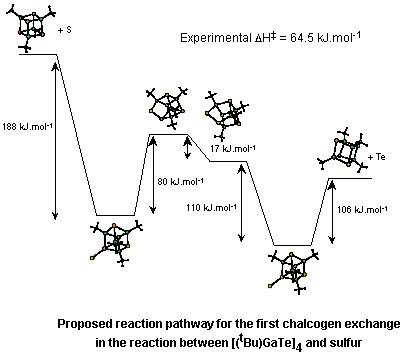
- Reaction of
[(tBu)GaTe]4 and elemental sulfur or
selenium, results in the stoichiometric formation of the
appropriate cubane, [(tBu)GaE]4 (E =
S, Se), and metallic tellurium (Organometallics, 1998,
17, 5310). Each of the intermediate cubane compounds,
[(tBu)4Ga4ExTe4-x] (x = 0
- 4; E = S, Se) has been characterized. The rate of the
chalcogenide exchange is not only dependent on the chalcogen but
also the allotropic form of the chalcogen. The chalcogen exchange
reaction is first order with respect to the cubane, and the
DH
 and DS
and DS have been determined. The exchange reaction is heterogeneous in
nature and involves the activation of the cubane via surface
absorption followed by cage opening.
have been determined. The exchange reaction is heterogeneous in
nature and involves the activation of the cubane via surface
absorption followed by cage opening.
Top of
Page
Siloxane cleavage
- We have shown that the products from the
cleavage of poly(diorganosiloxanes) with AlMe3 are
dimeric aluminum siloxides resulting from methyl transfer from
aluminum to silicon (Organometallics, 1990, 9,
2137). An extension of this concept involves the reaction of
R2AlH with cyclic siloxanes leading to rupture of the
silicon-oxygen framework and the formation of aluminum
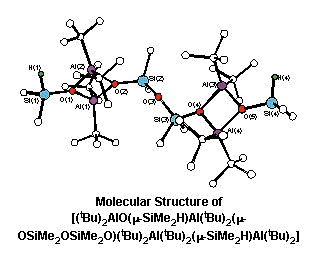
polysiloxides (Organometallics, 1999, 18, 5395).
Some examples of the unusual compounds formed by this route
include:
R2Al(OSiMe2H)(OSiMe2OSiMe2H)AlR2
(R = tBu, iBu),
Me2Al(OSiMe2H)AlMe2(OSiMe2O)Me2Al(OSiMe2H)AlMe2,
(tBu)2Al(OSiMe2H)(OSiMe2OSiMe2OSiMe2H)Al(tBu)2,
R2Al(OSiMe2H)AlR2(OSiMe2OSiMe2O)R2Al(OSiMe2H)AlR2
(R = tBu, iBu). The reaction pathway for the
cleavage of polysiloxanes has been explored.
Top of
Page
Lewis Acid Properties of Group 13 Metal
Compounds
- Activation of Small
Molecules
- Our study of the activation of small
molecules by Group 13 Lewis acids was initiated by the study of
the p-face
selectivity of coordinated ketones to nucleophilic addition. In
addition to the importance of aluminum-oxygen p-bonding
we noted that the reduction potential of the ketone is reduced by
up to 1 V upon coordination (J. Am. Chem. Soc., 1990,
112, 3446). This result led to a general study of the
interaction of organic carbonyls with sterically crowded aryloxide
compounds of aluminum (Organometallics, 1990, 9,
3086). A unique organic
transformation that takes place on sterically demanding aluminum
alkyl compounds is the direct conversion of an aldehyde to a
ketone (Tet. Lett., 1990, 31,
323).
-
- As part of our study on the hydrolysis
mechanism we have investigated alcohol and secondary amine
complexes of tri-tert-butyl aluminum. The observation of
enhanced stability through intra-molecular hydrogen bonding (J.
Chem. Soc., Dalton Trans., 1997, 3129) offers an insight into
the activation of an alcohol (or water) upon coordination to a
Lewis acid. The formation of the Lewis acid-base adduct activates
the a-proton by increasing its acidity, as measured by a decrease
in its pKa by about 7. However, the aluminum alkyl is
deactivated upon coordination of a Lewis base. This has led to our
proposal of an intermolecular mechanism for the hydrolysis
(solvolysis) of aluminum alkyls.
- Activation of
Metals
-
- In contrast to the well known reactivity of
Group 13 halides, the Lewis acidic nature of Group 12 halides, in
particular those of mercury, has been much less studied. However,
the chemistry of mercury(II) salts with aromatic hydrocarbons is
well developed and Hg...arene complexes are well
established as important intermediates, although simple complexes
have only been characterized spectroscopically. Based on the
possibility that Group 13 halide Lewis acids could "activate"
other weaker Lewis acids we have investigated the effect of
AlCl3 and GaCl3 on the stability of
Hg...arene complexes.
-
- The reaction of HgCl2 with two
molar equivalents of MCl3 (M = Al, Ga) in a substituted
aromatic solvent (C6H6-xMex) yields a
colored solution, from which crystalline material may be obtained
in moderate to high yield of
[Hg(arene)2(MCl4)2] for
C6H5Me, C6H5Et,
o-C6H4Me2, and
C6H3-1,2,3-Me3 (Angew. Chem.
Int. Ed., 2000, 39, 4117) In contrast, reaction of
HgCl2 with two molar equivalents of AlCl3 in
benzene, m-C6H4Me2,
p-C6H4Me2 yields liquid
clathrates.

- Each toluene in
[Hg(arene)2(MCl4)2], is
bound in a highly asymmetric h2
manner with the shortest Hg-C (ca. 2.3 - 2.4
Å),
being significantly shorter than observed for the intra-molecular
coordination discussed above (ca. 3.2 Å).
The stability of the Hg...arene interaction is as a consequence of
the activation of the mercury by the Group 13 halide.
-
- Solution NMR could not be obtained for
[Hg(arene)2(MCl4)2]. For
example, dissolution of the toluene complexes in
C6D6 results in the rapid quantitative
formation of C6D5Me and
C6D5H. It is important to note that if
C6H5Me and C6D6 are
mixed in the presence of < 0.1 mol% of
[Hg(arene)2(MCl4)2]
complete scrambling of the aromatic hydrogen/deuteriums occurs;
indicating that the H/D exchange reaction is
catalytic.
Top of
Page
Alumoxanes: Opening the Black
Box
- Redefining their
Structure
-
- Methylalumoxane (MAO), the product from the
hydrolysis of AlMe3, has significant industrial
application without industry having a clear picture as to what the
structure of MAO is! We set out to develop a cohesive view of the
structure of MAO by the study of the tert-butyl
analogs.
-
- Our isolation of the tert-butyl
alumoxanes, [(tBu)AlO]n (n = 6, 7,
8, 9, 12), was the first step towards the structural
characterization of MAO.
-
-
- Redefining their Mode of Activation:
Latent Lewis Acidity
-
- Based upon conventional wisdom, the cage
alumoxanes, [(tBu)AlO]n should be
inactive as co-catalysts with Cp2ZrMe2,
while
[(tBu)2Al{OAl(tBu)2}]2
would be expected to be an active co-catalyst, however, the
obverse is true: the electron precise cage compounds are active
co-catalysts with metallocenes (J. Am. Chem. Soc., 1995,
117, 6465). Spectroscopic characterization of the
catalytically active complex,
[Cp2ZrMe][(tBu)6Al6O6Me],
has led to our proposal that alumoxane's activity is derived from
their "latent Lewis acidity".
-
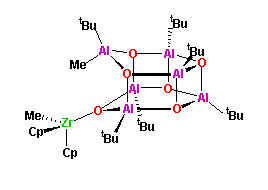
- Latent Lewis acidity is defined as the
ability of a electron precise molecule, e.g., a cage alumoxane, to
undergo cage opening, via heteroleptic bond cleavage, to
generate a Lewis acidic site. For a given bond type (i.e., an Al-O
dative bond in alumoxanes) the relative magnitude of the latent
Lewis acidity is related to the relative strain present in the
cage. Thus, in general four-membered Al2O2
rings are more strained than there six-membered
Al3O3 homologues, and hence exhibit higher
latent Lewis acidity. Based upon the angular distortions of the
cage atoms from an ideal geometry a semi-qualitative value for the
latent Lewis acidity may be obtained, allowing a prediction of the
relative reactivity of a series of alumoxane cage structures
(Organometallics, 1996, 15, 5514).
-
- Top of Page
-
-
- New Catalysts
-
- Once an understanding is obtained of the
structure and reactivity of alumoxanes, new catalyst systems may
be developed: alumoxanes as co-catalysts in palladium catalysed
co-polymerization of carbon monoxide and ethylene
(Organometallics, 1996, 15, 2213 and
Macromolecules, 1996, 29, 1110) and the latent Lewis
acid catalyzed polymerization of [R,S]-b-butyrolacetone
(J. Chem. Soc., Chem. Commun., 1997, 2183). Furthermore,
routes to highly active alumoxanes may be developed
(Organometallics, 2001, 20, 460).
-
-
- Group 13 Compounds as
Ligands
-
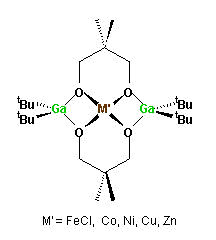
- We have recently started to explore the use
of Group 13 compounds as ligands to transition and main group
metals. Initial results are aimed at using gallium-(neopentane
diol) compounds (J. Chem. Soc., Dalton Trans., 2000, 2151)
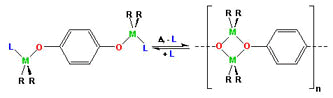
as cryptan ligands to transition metals.
-
- As part of our work in understanding the
mechanism by which borate anion cross links guar polymers, we
have demonstrated that borate moieties act as ligands to Group 1
metals (J. Chem. Soc., Dalton Trans., 2000,
3100).
-
Top of
Page
- Return
to Research in the Barron Group
Return
to Barron Group Home Page